
TGA's future business processes on complementary medicines open for discussion

A standardised process for pre-market assessments of complementary medicines with legislated timeframes and fee structures could be on the way.
As part of the continuing reform of the Australian therapeutic goods regulatory framework, the Therapeutic Goods Administration (TGA) has released a further consultation paper, seeking the views of consumers, health professionals and sponsors on the development and implementation of a range of proposed business processes aimed at improving the regulation of complementary medicines in Australia. Responses are requested by 7 November 2017.
Broadly, comments are invited on the following issues:
- business processes for pre-market assessment including legislated assessment timeframes and proposed fee structures for applications requiring evaluation;
- the introduction of risk-based application categories for the pre-market evaluation of complementary medicines;
- criteria to apply to the acceptance of reports from comparable overseas regulators and sources of evidence for de novo assessment; and
- strategies to enhance the post-market monitoring and compliance scheme for listed medicines.
Business processes for pre-market assessments including legislated assessment timeframes and proposed fee structures for applications requiring evaluation
The TGA is proposing to introduce a standardised process for pre-market assessments with legislated timeframes and fee structures. The TGA has illustrated the proposed process:
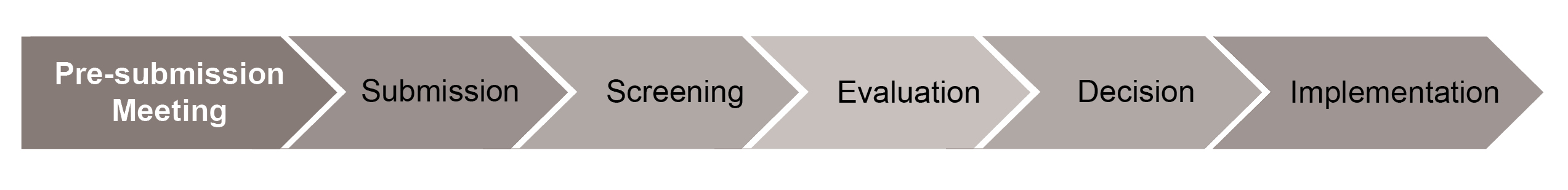
Complementary medicines listed in the "standard" manner, that is, that use only permitted indications, will continue to be listed on the basis of self-assessment/certification.
Each step of the process is detailed further as part of the consultation. Notably:
- an application fee and evaluation fee will apply. The application fee is payable on submission, and purportedly reflects the administrative costs associated with an application, whereas the evaluation fee will cover the cost of assessing the supporting information in an application. The latter is only payable at "Screening", that is once an application is deemed to meet the minimum requirements to proceed to "Evaluation";
- "Evaluation" may involve requests for information seeking further clarification. These requests will not however afford an applicant an opportunity to supply information that should have been originally included; and
- as part of the "Decision", the delegate will consider the evaluation report as well as any advice provided by an advisory committee and any subsequent comment provided by the applicant.
In support of this process a new fee structure is proposed, reflective of the level of risk of a new or varied complementary medicine and the level of scrutiny required to be undertaken by the TGA for the purposes of the pre-market assessment. At this stage, the TGA has provided initial estimates of the proposed Application and Evaluation fees. The proposed Application fees range from $430 for a listed assessed medicine in the lowest applicable category to $2,770 for a new registered complementary medicine in the highest category, while the proposed Evaluation fees for the same goods range from $1,640 to $35,500.
The proposed timeframes for each of the various assessment pathways range from:
- 70 to 180 working days for complementary medicine ingredients;
- 45 to 150 working days for assessed listed medicines; and
- 45 to 210 working days for registered complementary medicines.
The TGA is seeking feedback on these proposed business processes for pre-market assessments including both the proposed fee structures and legislated timeframes.
Risk-based application categories
The TGA also proposes the introduction of risk-based application categories in the pre-market evaluation of complementary medicines. It is proposed that these application categories will sit within each of the pre-market assessment processes to apply to complementary medicines, namely:
- new ingredients to be used in listed medicines;
- assessed listed medicines; and
- registered medicines.
It is envisaged that applications submitted in the lower risk categories will require less supporting information.
The TGA is seeking feedback on the proposed categories underlying each pre-market assessment as part of this consultation.
Criteria for acceptance of reports from comparable overseas regulators and sources of evidence for de novo assessments
The TGA has detailed the criteria it proposes using to determine whether reports from comparable overseas regulators are able to be relied upon by sponsors for the purposes of applications for assessment. The criteria have been designed to be applied through a two-stage process:
- firstly, the preliminary criteria that will be used to determine if there is sufficient similarity between the TGA and the overseas regulator; and
- secondly, the parameters that the TGA will consider to determine the suitability of evaluation reports from overseas regulators at the time of submission.
The TGA has also detailed the criteria it proposes be applied to alternative sources of evidence for de novo assessments.
The TGA is seeking feedback on both the criteria for acceptance of reports from comparable overseas regulators and sources of evidence for de novo assessments, which are set out in further detail in the consultation paper.
Enhanced post-market compliance monitoring scheme for listed medicines
The TGA has expressed concerns about the level of non-compliance by sponsors of listed medicines and has proposed three strategies to address this issue:
- Targeting of non-compliant sponsors. The TGA has noted that currently sponsors may withdraw their product from the ARTG following a request for information for a compliance review, only to later relist their medicine so as to avoid a possible negative finding. The TGA proposes disincentivising this behaviour by targeting such sponsors for post-market compliance review for multiple listed medicines, at least until such time as, in the opinion of the TGA, the sponsor's behaviours and actions improve. The TGA also proposes penalising sponsors who repeatedly demonstrate a clear intention to circumvent their obligations. The example given is the use of infringement notices for repeat offenders;
- Improving transparency about compliance review outcomes. The TGA proposes providing more detailed information about products that are the subject of a compliance review, including the compliance issues identified during any such review, the actions taken by the sponsor in response; and/or if the product is cancelled, details as to the reasons why the product was cancelled. The TGA acknowledges that this will involve a balancing act between consumer protection and the protection of commercial information and procedural fairness; and
- Education and resources for product sponsors. The TGA proposes educating sponsors with better tools and resources to improve their understanding of their regulatory obligations.
The TGA is seeking feedback on the measures proposed above, including the educative tools that may result in greater compliance.
Next steps
This consultation process closes on 7 November 2017. If you would like to know more, or want help with your submission, please contact us.
Get in touch
